Uranium
What is Uranium
Uranium Geology
Uranium Deposits
Uranium Resources
Uranium Mining
Uranium Logs
What is Uranium?

Uranium was formed by supernova events billions of years ago, prior to the formation of our solar system. Today, its slow radioactive decay provides the main source of heat inside
the earth's crust. Uranium is a heavy metal that can be used as an abundant source of concentrated energy. It usually occurs as an oxide; an example of a uranium
mineral is uraninite (UO2). Uranium metal is about 60 percent denser than lead and almost as dense as gold. It occurs in trace amounts nearly everywhere on the planet, even in seawater.
Uranium was discovered in 1789 by German chemist Martin Klaproth in an ore known as pitchblende. It was named after the planet Uranus, which had been discovered eight years earlier.
Isotopes of Uranium
Naturally occurring uranium consists primarily of three isotopes (atoms with the same number of protons but a different number of neutrons). The most abundant uranium isotope is 238U
(which has 146 neutrons and 92 protons in the nucleus), followed by 235U (which has 143 neutrons and 92 protons in the nucleus) and trace amounts of 234U (which has 142 neutrons and
92 protons in the nucleus). Other isotopes of uranium are known but very rare, and usually short lived. Some can be made artificially, such as 239U, which is produced during the process of converting
238U to 239Plutonium, while 232Thorium can be converted to 233U.
All isotopes of uranium are radioactive, meaning their nuclei are unstable and will decay over time. The rate of decay is known as the radioactive half-life. 238U has the longest half-life at 4.47 billion years,
and is considered the most stable. 235U has a half-life of 703 million years, while 234U has an even shorter half-life of 245,300 years. Because 234U and 235U have
relatively short half-lives, the relative abundance of these isotopes has decreased compared to 238U since the formation of the Earth.
Uranium Decay Series
When a uranium nucleus decays, it emits radiation (in the form of energy or particles) and the number of particles in the nucleus changes. A change in the number of protons transforms the uranium atoms into other elements, which we call decay or daughter products.
Uranium isotopes must undergo multiple decay events before reaching a stable form in a process known as a decay series.
The decay series of 238U produces unstable 234U as an intermediate daughter product during this process, and ultimately ends by forming stable 206Lead. The 235U decay series ends when the stable 207Lead isotope is produced.
Uranium atoms decay slowly by emitting alpha particles. An alpha particle emitted from the uranium nucleus is positively charged and made up of two protons and two neutrons, which is physically and chemically identical to a
helium nucleus. Daughter products created during the 238U and 235U decay series can also emit beta particles and gamma radiation.
Based on rates of decay, geoscientists can use the different uranium decay series to determine the ages of rocks and geologic events.
Clean Energy Source
Nuclear energy produced from uranium is recognized as a practical, inexpensive, and clean source of energy. A typical 1,000-megawatt
reactor can provide enough electricity for a modern city of up to 1 million people. Nuclear power is reliable, and power plants emit no carbon dioxide.
The emissions coming from the massive towers of a nuclear plant are actually water vapor. Nuclear power boasts the best capacity factor of all forms of electrical generation.
The capacity factor, the percentage of time a power point is online and actively generating electricity, is a measure of reliability. A nuclear power plant can run at above 90 percent capacity.
A coal-fired plant runs at about 64 percent, a natural gas power plant at 43 percent, and a hydroelectric plant at 40 percent.
![Nuclear Power Plant [Credit: WSGS] Power Plant](../images/energy/power-plant.png)
In a nuclear-fueled power plant, water is turned into steam, which drives turbine generators to produce electricity. The main difference between a nuclear power plant and a coal- or
natural gas-fired power plant is the source of heat. At a nuclear power plant, the heat to make the steam is created when uranium atoms split by a process called fission. There is no
combustion in a nuclear reactor, unlike at fossil fuel power plants.
Nuclear Fission
235U is the only uranium isotope considered fissionable. When the nucleus of a 235U atom is struck by a moving neutron, it splits in two. This split, known as a fission reaction, releases
energy in the form of heat and radiation, plus an additional two or three neutrons are expelled from the nucleus. If enough of these expelled neutrons strike the nuclei of other 235U atoms
nearby and causes them to split, releasing even more energy and neutrons, a fission ”chain reaction” can be achieved. When this happens repeatedly, many millions of times, a very large amount
of heat is produced from a relatively small amount of uranium. This is the process harnessed at nuclear power plants to produce electricity.
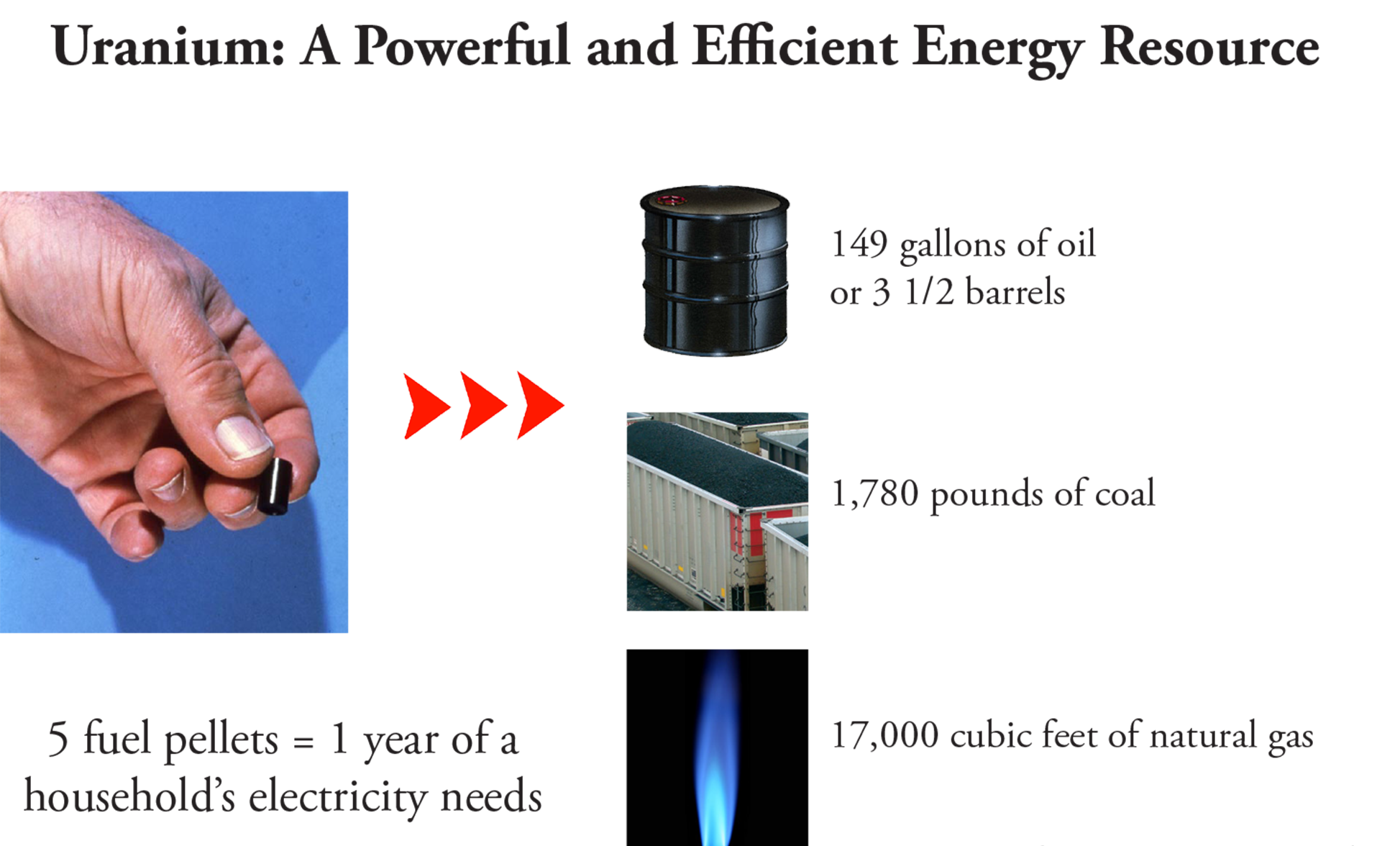
Fuel
The fuel used in a nuclear plant must contain enough fissionable material to create a chain reaction. All of the nuclear power plants in the U.S. require uranium fuel with a higher concentration of the 235U
isotope than is found in nature. These plants use low-enriched uranium fuel, which contains 3-5 percent 235U (compared to 0.7 percent 235U found in naturally occurring uranium).
Advanced nuclear reactors, such as the Natrium reactor scheduled to be built at the Naughton Power Plant near Kemmerer, WY, require fuel with a concentration of 5-20 percent 235U. Fuel with this range
of 235U content is known as High-Assay Low-Enrichment Uranium (HALEU).
There are multiple steps required before uranium ore can be enriched and fabricated into fuel. After the ore is mined and milled into yellowcake (uranium oxide, commonly U3O8), it is transported to a conversion
plant, where it is converted to uranium hexafluoride (UF6) gas. This gas is then transported to a fuel enrichment facility, where it is enriched in 235U. Afterwards, the enriched uranium gas is transported to
a fuel fabrication facility, where the enriched gas is converted into solid fuel rods, which then get shipped to nuclear power plants.
Helpful Links
The US Nuclear Regulatory Commission (NRC), https://www.nrc.gov/, provides information about nuclear reactors, fuel, and the facilities that mine, process, and utilize it.
Definitions of nuclear materials: https://www.nrc.gov/materials.html
Stages of the nuclear fuel cycle: https://www.nrc.gov/materials/fuel-cycle-fac/stages-fuel-cycle.html
Uses of nuclear energy and more: World Nuclear Association, Information Library
Source: World Nuclear Organization
Contact:
Kelsey Kehoe, kelsey.kehoe@wyo.gov